Miracles in Tin Stripping
by Doug McKesson, RD Chemical Company
My boss laughed when I proposed this title for this paper, and then stopped when he realized that I was serious when I made the proposal. And I hope that you will agree, when I am finished, that although it stretches the matter a bit, this paper discusses an approach that does seem almost miraculous.
As we are all aware, the most popular method of processing outerlayer printed circuit boards in the United States and Europe is by pattern plating. Pattern plating employs photoresist to act as a plating resist for tin or tin/lead. The tin or tin/lead electroplate then acts as the etch resist. After etching, the remaining copper is covered by the electroplated tin or tin/lead. The next step is to remove the tin or tin/lead, leaving bare copper circuitry behind.
Today’s tin or tin/lead stripping solutions are almost always proprietary mixtures, but their compositions are quite similar, regardless of vendor. The typical composition is as follows
20% (w/w) Nitric Acid
5% (w/w) Ferric ion Fe+++
<1% (w/w) Anti-tarnish
<1% “Suspending Agent”
The typical spent tin stripper will contain the following
12 - 14% (w/w) Nitric Acid
5% (w/w) Ferric ion Fe+++
<1% (w/w) Anti-tarnish
<1% “Suspending Agent
150 g/L Tin (or 90 g/L tin and 60 g/L lead)
The ultimate goal at this point in this process is to strip the electroplated tin or tin/lead. To facilitate this mechanism, the tin or tin/lead must be oxidized. Oxidation is the first step in any metal stripping process, and the purpose of which is to remove electrons from a substance. In the case of stripping tin or tin/lead, the oxidizer is nitric acid.
Unfortunately, we are all too familiar with the oxidation, or tarnishing of copper. This is a slightly different result, but the mechanism is the same. Elemental copper is an extremely active substance, meaning it is very easily ionized. . The result is copper oxide, or tarnish.
When exposed to air, copper gives up two electrons and the oxygen gains two electrons, as can be seen by the reaction below
Cu0 + O —» CuO
Reduction is the reverse of oxidation, and means giving electrons to a substance. When a substance is oxidized, the oxidizing agent is reduced. Since the metal in the above reaction is oxidized by atmospheric oxygen, the oxygen is reduced by gaining those same electrons. Reduction is what occurs to the chemical that oxidizes the metal being stripped. This is hard to follow, but when oxygen oxidizes (removes electrons from the metal), the oxygen is reduced in the process, gaining those same electrons. Wherever oxidation occurs, reduction must also occur in the same reaction, which is known as an oxidation-reduction reaction. The reaction for tin stripping below should illustrate the concept of oxidation-reduction.
2Sn0 +2HNO³ —» 2SnO² + N²O + H²O
Notice that each mole of tin was oxidized, losing four electrons, and each mole of Oxygen was reduced, gaining two electrons each for a total of four. Water and nitrous oxide are produced as by-products of the oxidation-reduction reaction.
All pure elements, like tin or copper, are neutral, meaning they do not carry any charge, and they have the same number of electrons as protons. For any metal to go into solution the metal must carry a positive charge. In the tin stripping reaction, removing electrons with an oxidizing agent gave the elemental tin a positive charge.
Tin has three different ionic charges or oxidation states that can occur:
1. Tin metal Sn0 (neutral)
2. Stannous Salts, Sn++ (missing 2 electrons)
3. Stannic Salts, Sn++++, (missing 4 electrons)
In reference to the stripping process, it is absolutely crucial that the tin is oxidized completely to the stannic (Sn++++) form. Once the metal has been oxidized, it must then be dissolved into solution. Unfortunately, most stannic salts are insoluble in water. This includes the stannic oxide formed during the stripping reaction. In fact, stannic oxide is not even soluble at any concentration of nitric acid. In order to keep the stannic oxide from precipitating out, it must be “held” in the liquid phase, or dispersed, by the suspending agent mentioned earlier. The suspending agent keeps the stannic oxide in solution by creating a fine dispersion of stannic oxides within the stripper solution. This creates an environment where the stripper solution does not separate or “sludge out”, so it will not clog the spray manifolds or nozzles, even though it is not a true solution.
Ideally, this system should strip Tin preferentially over copper. This is, however, quite difficult since copper is much more readily oxidized than tin. The addition of inhibitors keeps the copper oxidation under control, leaving the unprotected tin under susceptible to oxidation by the nitric acid.
The typical industry standard tin and tin/lead strippers contain around 150 g/L tin (or combined tin and lead), in feed and bleed mode, and upwards of 200 g/L in batch mode. Depending upon the inhibitor system, typical strippers will contain around 1 – 2 g/L of dissolved copper as well. The real cost to the user, however, lies in the waste treatment of spent tin stripper.
Because of the peculiar nature of the tin dispersion, the system cannot be treated through conventional ion exchange, or basic metal flocculation methods. This means the spent stripper must be packaged and transported as a hazardous waste to a qualified disposal site for burial. Aside from the horrendous environmental detriment it causes, this process also runs the fabricator nearly $5 / gallon in disposal and transportation costs.
To make things even more agonizingly painful for the fabricator, there is potential monetary value in the form of stannic oxide dissolved in the spent stripper solution. There is approximately $2.90 worth of tin present per gallon of spent stripper solution. Obviously it would be in the fabricators best interest to be able to remove the tin values from the stripper solution, and convert it to a usable (and valuable) form.
No, RD Chemical is not boasting itself to be workers of miracles because they thought up the idea of removing tin from spent tin strippers. I would assume that most vertebrates in the PCB industry have bounced the idea around their heads once or twice. However, since spent tin or tin/lead strippers cannot be processed through normal waste treatment procedures, a viable alternative has not yet been developed.
This led us to the idea of electrowinning, which is arguably one of the more popular topics that have been discussed in the PCB industry over the past decade. Electrowinning is a widely used method for recovering heavy metals from spent process solutions prior to waste treatment. Electrowinning uses electroplating techniques specifically to recover metals from spent process solutions. This method of tin recovery seems to be a logical conclusion. However, the problem to date is that electroplating is only possible from electrolyte systems that are true solutions. Returning to our previous discussion, we remember that the spent stripper as received, is not a true solution, but a dispersion of stannic oxides within the solution. This is unfortunately one of the cardinal rules of electroplating, which means that the tin values cannot be recovered from this system without further treatment.
A New Approach
Research was conducted in an attempt to eliminate the expense of waste treatment of spent tin strippers. Specifically, it was the goal of our research to provide an alternative method of handling the spent tin stripper which would give the PCB industry a less expensive, and a more environmentally responsible approach. It was decided that electrowinning would be the optimum method for metal recovery from this system. Making the tin stripper system feasible for electrowinning implies that the recovery method could be configured to remove not only tin, but also all heavy metals, including the copper and lead. Furthermore, Electrowinning also returns the metal in a form that is reasonably pure, and thus readily accepted by refiners.
The key to electrowinning is that all the metals to be plated out must be in true solution, not merely dispersed particles, as is the dissolved Tin in the spent stripper. The Tin that is stripped in the common Nitric Acid based strippers is fully oxidized to Stannic Oxide, which is not soluble in the dilute Nitric Acid of the stripper, and is present as a disperse, insoluble particle. It was realized that if the metals are to be recovered via electrowinning, the electrolyte system of the spent tin stripper must be changed to dissolve the tin. In order to do this, the system must be changed from a dispersion to a solution. Specifically, the electrolyte must be changed to a form where the stannic oxide is soluble. This is a daunting task, to say the least, since the stannic oxide is soluble in very few systems.
The stannic oxide in the spent stripper however, is soluble in only two common electrolytes; hydrofluoric acid and strong caustic. Stannic oxide is not soluble in the dilute nitric acid of the stripper. We would all agree that although neither chemical system is fun to be around, Hydrofluoric Acid is an absolute nightmare. A caustic based recovery system was therefore the only alternate, which we chose as our approach. The first attempts were not promising, as the spent tin stripper would gel completely once addition of sodium hydroxide made the system slightly alkaline.
However, with a few months of development work, an additive was developed that would dissolve all the metals in a caustic solution, and create a free-flowing electrolyte from which all heavy metals could be plated out.
Most spent tin strippers contain on the order of 150-200 grams/liter of Tin (similar total quantities of tin and lead, when it is a tin/lead deposit being stripped) and about 1-2 grams/liter of dissolved copper.
When plating mixed metals, it takes considerable skill and manipulation of the system to get the metals to plate out at the same time. They instead default to plating out sequentially, one after the other. However, with a lot of research work and some considerable luck, the electrolyte additive was refined to cause the copper and lead to plate out ahead of the Tin. This ensures that after pH adjustment, the final solution would be legal to dump down most drains, without treatment, even if small amounts of tin are remaining in the electrolyte.
The tin recovery system uses sodium hydroxide to convert the insoluble stannic oxide to the soluble sodium stannate form, via the reaction below. Notice the tin remains in the Sn4+ (stannic) Oxidation State.
SnO² + 2NaOH —» Na²SnO³ + H²O
The make-up procedure works as follows:
- Spent tin stripper is transferred to a specially designed electrowinning system.
- 1 gallon of “TinCovery Additive T” is added to each gallon of spent stripper.
- The bath is then heated to 70° C to facilitate a reasonable plating rate.
- The bath is then plated at a carefully controlled current density of 8 A/ft2, until the tin content is depleted.
The optimum current density is approximately 10 amperes/ft2, but the exact current density will be defined by the design of the electrowinning cell. At the optimum current density, the electroplating reaction is as follows:
Na²SnO³ +H²O + 4e- —» Sn0 + O² +2NaOH
Excessively high current density will cause the tin to be converted to stannous Sn++ tin, via the following reaction:
Na²SnO³ + H²O 2e- —» Sn++ + O² +2NaOH
If the current density is raised beyond the optimum level, the chemical requirements as well as costs will increase, and the rate of tin removal will diminish drastically.
Tin concentration in the electrowinning cell is controlled by measuring the density (Baume’) and is used to monitor the progress of the electrowinning process. Figure 1 illustrates the relationship between specific gravity vs. metal concentration.
Final metal analysis should be performed by Atomic Absorption Spectroscopy to determine when the Tin has reached an acceptably low level, at which time the bath may be dumped.
Key ultimate benefit of this system is that when it has been determined that once the copper concentration has reached an acceptably low level (2 ppm in most municipalities), the bath is pH adjusted and may be put into most effluent systems without further treatment. Once the copper concentration (and lead, if applicable) is depleted, electrowinning is continued until the tin recovery rate reaches a practical minimum.
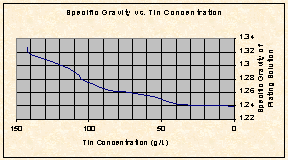
In Figure 2., a similar plot was created, showing the relationship between copper concentration and specific gravity. Although the scale is much smaller and it is more difficult to calculate copper concentration from the specific gravity, it can be concluded that the copper concentration is depleted once the specific gravity drops below 1.300.
The ultimate result of this development will assist the PCB industry in cutting the cost of tin stripping, while minimizing the amount of another hazardous waste currently being generated by PCB fabricators.