Rinsewater Quality….Hard Data
Doug McKesson, Dr. M.J. Wegener, RD Chemical Company
Introduction
In nearly every chemical process step in the PCB industry, rinsing is an immediate, and required process step. Rinsing is typically a crucial step following a chemical process, and is thought to be one that requires little or no attention to function properly. However, problems caused by ineffective rinsing are responsible for many rejects, as well as huge operating costs in the waste treatment department.
At first thought, rinsing is often defined as the removal of process solution from the work, or in the case of the PCB industry, a panel. This is true, if not absolutely true. Rinsing, in general, is not the complete removal of the contaminates, but rather a dilution of a process solution from the work (panel) down to “manageable” concentrations. With this definition in mind, it can be seen that complete removal of process solution from the work is very difficult, unnecessary, and essentially never accomplished in the PCB industry.
Is there an absolute standard which can be applied to rinsing? Or in other words, is there a standard we can use for all rinsing, to determine what are “manageable” concentrations of contaminants remaining on the work? The answer is, not really. What constitutes a “manageable” concentration is dependant upon three conditions:
- The type of contaminant.
- The tolerance of the following process step for the particular contaminant in question.
- The effect the residual contaminants have on the work.
Let us examine the contamination, or “drag-in” of an alkaline cleaner into a persulfate based microetch vs. the same cleaner dragged into an acid copper plating bath. Both baths are acidic, and some acidity will be neutralized by drag-in of alkaline residues. Depending upon the concentration of drag-in, the alkaline solution will have an almost negligible neutralization of the microetch. This is true because the acidity of the microetch is self-generated by the spontaneous decomposition of the persulfate, thus the decrease in acidity is, to some extent, automatically replenished.
When examining an acid copper plating bath, however, we know that this bath is extremely sensitive to contaminants, and can be ruined by the same level of contaminants that had no effect on a microetch. We can conclude that a level of contaminants from an alkaline cleaner that one process solution can tolerate may not be tolerable for a different process solution. Manageable levels of contamination would need to be determined for each specific process.
In both cases discussed above, effective rinsing will prolong bath life of process solutions down the production line. An effective rinsing system that effectively removes enough process solution can decrease chemical costs, lower reject rates, and ease the burden on waste treatment systems.
There are several myths associated with rinsing performance that deserve discussion. A common myth is that alkaline solutions are more difficult to rinse than acid solutions. As a consequence, we often see rinses following alkaline cleaners using hot water, and/or extremely long exposure times. Let’s examine these myths further.
These observations likely started due to difficulty of removing the slick feeling that an alkaline solution gives when in contact with the skin. In actuality, the slick feeling is never “rinsed” away, because the slickness is due to the fixed oils on your skin being converted to a soap by the alkalinity of the solution. What you wind up doing is converting this “soap” to a hard water scum when rinsing your hands in water, or to a fatty acid, when rinsing in an acidic solution. Since these oils are not found on copper, soldermask, laminate or other materials, rinsing alkaline solutions from printed circuit boards is no more difficult than rinsing acidic solutions.
Alkaline cleaners can also get blamed for poor rinsing because of the presence of foam generating surfactants in the formulations. The surfactants used in many alkaline cleaners generate large amounts of foam even at incredibly low concentrations. When a small amount of surfactant is dragged into a rinse tank, it can create a stable foam, even at concentrations that would not affect subsequent process steps. The cause of the foam, however, is not because the surfactants are not rinsed inadequately from the work, but because the presence of these surfactants in extremely minute amounts can cause substantial foam generation.
The perception that an alkaline cleaner would rinse poorly was further supported by the formulation chemistry followed by some chemical vendors during the infancy of the PCB industry. Some of the early alkaline cleaners were formulated using silicates. Calcium present in tap water was interacting with the silicates in these cleaners forming calcium silicate, which precipitated out of solution, and onto the work. Unfortunately, even extensive rinsing would not always help the situation, and rejects resulted. Thankfully the industry, and its suppliers, have grown up since that time, and silicates are no longer present in most of the cleaners used by the PCB industry.
Still another theory widely held throughout our industry reasons that if work is left in a rinse tank for a longer period of time, better rinsing will be the result. Let us apply the dilution model we discussed earlier, as a review of this information would prove this theory to be false.
When a rack of PCB’s is immersed in a rinse tank, the residual surface contamination is reduced to a practical minimum within 30 seconds, as the solution carried in on the surface of the work disperses into the rinse waters. A typical rinse tank, say of 100 gallons, with a water flow of 5 gallons per minute would decrease the concentration of the solution contaminants at a rate of only 5% for each minute that it remains in the rinse tank. Leaving the work in any longer would have virtually no effect.
It would be much more preferable to have brief exposure times in many (presumably cascade) rinse tanks, which will result in a better dilution rate of the contaminants, than a long exposure time in just a few rinse tanks.
There have been countless studies performed on rinsing mechanisms, and the literature is ripe with information on achieving high quality rinsing. Most studies are based upon multitudes of calculations of volume of water flow relative to number of rinse tanks in use1.
In some areas, cascade rinsing is currently held in high favor even though this method can substantially increase water consumption. In other areas, PCB fabricators are constantly concerned with amount of water consumed. Both the price tag attached to each gallon of water consumed, and the cost of waste treating the rinse waters are major factors. We see therefore, that the “more is better” attitude to improving rinse water quality cannot be acceptable across the board.
This brings us to the ultimate question. Namely, how can we further improve rinse quality, without increasing water consumption and waste generation?
One of the areas of rinsing that most engineers are aware strongly affect quality of rinsing, and quality of product, is the “hang” or drain time of a PCB above a process or rinse tank, in a rack line. Long hang times give excellent rinsing efficiency, by minimizing drag-in to the next tank, but excessive hang times can also create quality problems by allowing panels to dry, or to tarnish. Either condition can cause quality problems in subsequent process steps.
Since the above considerations are all, to some extent, known, it is simply stunning that there is no published data that allows any sort of calculation for optimum hang times.
Please allow me to introduce data obtained from several experiments performed at RD Chemical Company to try and determine if there exists an optimum time for a circuit board to drain.
First, with no industry standard, we needed to develop a basic laboratory protocol. After numerous starts without repetitive results, we settled on the following procedure which gave us results that have proven to be reasonable and that could be charted.
Experimental Procedure
Equipment:
Electronic balance
Polypropylene tank (2.5” x 30” X 20”) with straps
Eight foot 2 X 4
Stainless steel hooks
Plastic coated wire
Stop watch
Voice recorder
The experimental setup was composed of an electronic balance, the older variety where the numbers flashed by during the weighing, which was placed on a board above the ground. A hanger was balanced on the scale, the ends of which attached to the top of a circuit board at each side using plastic coated wire. The hanger, chains and board were then tared, i.e in air they had zero weight.
The polypropylene tank containing water was then elevated by hand so that the water completely covered the circuit board.
At a given signal, the tank was lowered rapidly without allowing the board to touch the sides of the tank.
As soon as the water was no longer in contact with the PCB, a stopwatch was started and the readings begun. The first positive reading was then taken as the initial weight of the water on the board and the change in weight was recorded orally.
After the first 15 to 20 seconds, the time as well as the weight could be recorded. The initial times had to be estimated by timing the playback of the recorder.
Due to the sampling interval of the balance, and how quickly the numbers changed, the first few seconds gave inconsistent numbers, so that the real weight of the water on the board could not be accurately determined.
The change in weight of the board (loss of water) was plotted versus time.
Results
Upon evaluation of the initial results, it became evident from the shape of the plot, that the exact weight of the water to start was irrelevant considering the objectives of our experiment.
Several measurements were made on several boards, including two outer layers with solder mask and solder plated, and one oxide-coated inner layer with no holes. The rate of draining was measured for boards held level and for boards slightly canted (15-20).
In another phase of the experiment, the effect of added surfactant was explored.
The graph for the loss of water with time is shown in Fig. 2. Perhaps not surprising to many of you, we could have indicated that this was the graph for either of the two other boards, or when the board was level or when the board was canted, or even when the surfactant was present. They were all very similar in appearance.
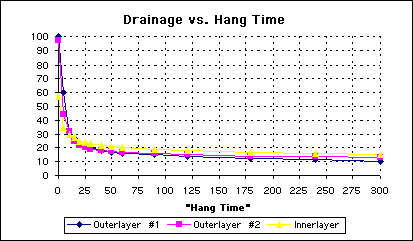
Figure 1 - Water Drain from PCB’s Over Time
The striking feature of the graph is not seen so much in the numbers compared to the prevailing shape. A replot of this data with semilog scale (See Figure 2.), clearly shows the two linear regions.
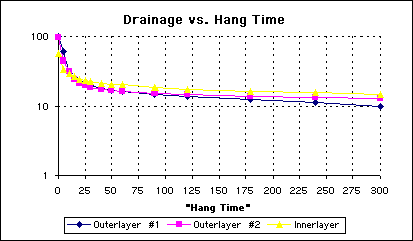
Figure 2 - Semilog Plot of Water Drain Over Time
There appears to be two different phenomenon occurring when water drains from the board. Note from the figures above there is a very rapid loss of the majority of solution, followed by a fairly slow loss of the remaining solution. Each mechanism exhibits a linear or nearly linear behavior with a transition regime where both mechanisms seem to come into play. From examining these graphs, note that the optimum time for a board to hang over a tank to drain is about 30 seconds. While there is not a sharp break, at 30 seconds the transition to the slower draining phenomenon is nearly complete. Now thirty seconds is not the magic number, but if the panels were allowed to hang over the process tank for less than 20 seconds, the dragout could become significant and VARIABLE with small changes in time.
Interestingly, the shape of the curve, when weight loss is plotted against time, does not change when surfactant is added to the water (See Figure 3).
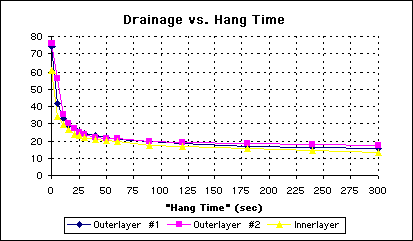
Figure 3 - Plot of Drainage Over Time for an Aquous Surfactant Solution
A semilog plot of the same data confirms that the gaddition of the surfactant has negligible effect on the drainage rate (See Figure 4).
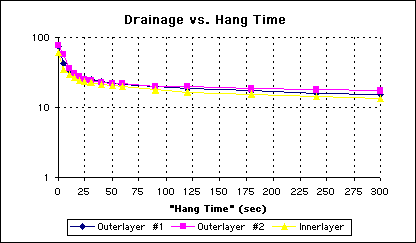
Figure 4 - Semilog Plot of Drainage Over Time For Aqueous Surfactant Solution
On outerlayer panels, canting the board slightly during draining did not improve the drainage rate. On innerlayer panels, however, the effect was quite noticeable (See Figure5). The transition region for the innerlayer is narrowed when the board was canted. We did not see this with the outer layer boards, where the optimum hang time remained the same.
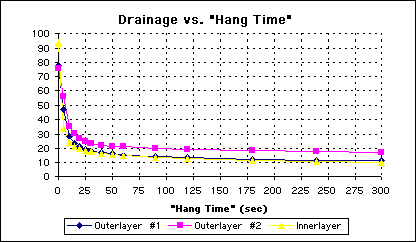
Figure 5 - Plot of Water Drainage Over Time From Canted Panels
Observing the semilog plot for the canted panels again confirms that the narrowing of the transition region, and the same 30 second optimum hang time (See Figure 6).
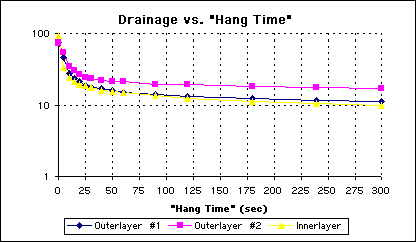
Figure 6 - Semilog Plot of Drainage Over Time from Canted Panels
Based upon the above data, an equation was derived to relate a given hang time to the amount of fresh rinsewater flow necessary to dilute contaminants to a given concentration, which in this case will be to 0.1% and 0.01%.
The equation is found below.
Required Rinse Water Flow (gpm) =
Vd X As X Rd
Where:
Vd = Volume of Dragout per unit area
As = Surface area processed per minute
Rd = Desired Dilution Ratio
The calculations were based upon a hypothetical innerlayer oxide line with two 25 gallon cascading rinse tanks, and 10 innerlayer panels per rack. For simplification, the carry-over of rinsewater from the second rinse tank to the first rinse tank is assumed to be zero.
The water flow rates for the above scenarios can be found in the Table 1.
Table 1 - Calculated Rinsewater Flow Rates for Hypothetical Process Line for Two Targeted Contaminant Concentrations
|
Hang Time (s)
|
0.1%
|
0.01%
|
|
5
|
0.94
|
2.9704
|
|
10
|
0.68
|
2.1488
|
|
30
|
0.5
|
1.58
|
|
60
|
0.4
|
1.264
|
|
120
|
0.3
|
0.948
|
The calculated flow rates were then plotted versus hang time (See Figure 7). Not surprisingly, the graph has the same behavior as the previous graphs, showing again that a hang time of around 30 seconds, will minimize flow rate of the rinsewaters necessary to bring the concentration of the contaminants down to the targeted levels.
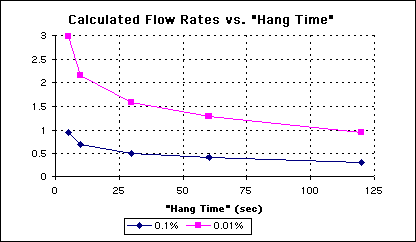
Figure 7 - Plot of Rinsewater Flow Rates vs. Hang Time for Targeted Contaminant Levels.
Discussion
Although an absolute rinsing standard for all chemical processes in the PCB industry is unattainable, we have shown that rinsing can be optimized by the use of many rinse tanks, and the appropriate hang times. While the manageable levels of contaminants vary for different processes, we know that a good dilution rate equates to good rinsing across the board. Determining the appropriate hang time of a panel above the process solution helps to improve the dilution rate, without incurring more water consumption. Results of our research showed not only that increasing the hang time can decrease rinsewater usage, but showed that we can determine an optimum hang time. Beyond the optimum hang time, allowing the panel to drain longer, further benefits diminish. The results of this optimization will give increased quality of final product with fewer rejects, decreased chemical costs, and lowered burden on waste treatment systems.
References
- T. Mooney, The Art and Science of Water Rinsing, Metal Finishing Handbook, 1996., pp 135